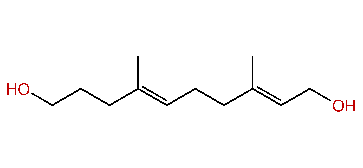
| (E,E)-3,7-Dimethyl-2,6-decadien-1,10-diol |
| Formula: | C12H22O2 |
| CAS#: | 24048-35-9 |
| MW: | 198.3 |
[MS]
[ NMR ]
[Behavioural function]
|
|
Reference(s) for synthesis of (E,E)-3,7-Dimethyl-2,6-decadien-1,10-diol [3me7me-E2E6-1,10-diol]
| Katzenellenbogen, J.A., and Cristy, K.J. 1974. Stereoselectivity of the rearrangement of allylsiloxyvinyl ethers. A highly stereoselective synthesis of a diol found in the pheromonal secretion of the queen butterfly. J. Org. Chem. 39:3315-3318. |
| |
| Masaki, Y., Sakuma, K., and Kaji, K. 1980. A new stereoselective synthesis of a terpenoid diol component of the pheromonal secretion of the queen butterfly. Chem. Lett. 1061. |
| |
| Masaki, Y., Sakuma, K., and Kaji, K. 1985. Regio- and stereoselective terminal allylic carboxymethylation of gem-dimethyl olefins. Synthesis, of biologically important linear degraded terpenoids. Chem. Pharm Bull. 33:1930-1940. |
| |
| Miles, D.H., Loew, P., Johnson, W.S., Kluge, A.F., and Meinwald, J. 1972. A short stereoselective synthesis of some terpenes from the pheromonal secretion of the queen and Monarch butterflies. Tetrahedron Lett. 30:3019-3022. |
| |
| Nystroem, J.-E., and Baeckvall, J.-E. 1983. Synthesis of the major pheromonal component of the Monarch butterfly (Danaus plexippus) via palladium-catalyzed 1,4-functionalization of isoprene. J. Org. Chem. 48:3947-3950. |
| |
| Odinokov, B.N., Kukovinets, O.S., Sakharova, N.I., and Tolstikov, G.A. 1989. J. Org. Chem. USSR (Engl. Transl.). 25:25-28. |
| |
| Zakharkin, L.I., and Petrushkina, E.A. 1984. Stereospecific synthesis of (E)-3,7-dimethyl-2-octene-1,8-diol from a telomer of isoprene with diethylamine. Zh. Org. Khim. 20:2073. |
| |
|







