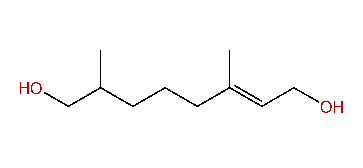
| (E)-3,7-Dimethyl-2-octen-1,8-diol |
| Formula: | C10H20O2 |
| CAS#: | 33150-33-3 |
| MW: | 172.27 |
[MS]
[ NMR ]
[Behavioural function]
|
|
Reference(s) for synthesis of (E)-3,7-Dimethyl-2-octen-1,8-diol [3me7me-E2-octen-1,8-diol]
| Araki, S. and Butsugan, Y. 1984. Transition-metal-catalysed Grignard reaction of secondary allylic phosphates. J. Chem. Soc. Perkin Trans. 1:969-972. |
| |
| Ferroudt, D., Gaudin, J.M., and Genet, J.P. 1986. Bis(arylsulfonyl)methane: a versatile synthon in pheromone synthesis. Tetrahedron Lett. 27:845-846. |
| |
| Fujisawa, T., Sato, T., Kawara, T., and Noda, A. 1982. A novel synthesis of (E)-3,7-dimethyl-2-octene-1,8-diol secreted by the African monarch using the ring-opening reaction of alpha-methyl-ß-propiolactone. Tetrahedron Lett. 23:3193-3194. |
| |
| Gramatica, P., Giardina, G., Speranza, G., and Manitto, P. 1985. Baker's yeast hydrogenation of carbonyl activated double bonds. Enantioselective synthesis of the (S)-form of the dihydroterpenediol secreted by Danaus chrysippus and of a pheromone of Callosobrachus chinensis L. Chem. Lett. 1395. |
| |
| Meinwald, J., Thompson, W.R., Eisner, T., and Owen, D.F. 1971. Pheromones. VII. African monarch: major components of the hairpencil secretion. Tetrahedron Lett. 12:3485-3488. |
| |
| Morizur, J.P., Bidan, G., and Kossanyi, J. 1975. Insect chemistry III. Use of the photochemical Norrish Type I reaction to the synthesis of the dihydroterpenediol secreted by the African monarch butterfly. Tetrahedron Lett. 16:4167-4170. |
| |
|







