|
|
�
�
|
| |
Compound - 4me-heptan-3-ol [4-Methylheptan-3-ol]
|
| |
| |
�
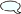
 �
�
|
| Formula: | C8H18O |
| CAS#: | 14979-39-6 |
| MW: | 130.23 |
[MS]
[ Kovats ]
[Behavioural function]
|
|
Reference(s) for synthesis of 4-Methylheptan-3-ol [4me-heptan-3-ol]
| Einterz, R.M., Ponder, J.W., and Lenox, R.S. 1977. The synthesis of 4-methyl-3-heptanol and 4-methyl-3-heptanone. J. Chem. Educ. 54:382. |
| |
| Frater, G. 1979. Stereospezifische synthese von (9+)-(3R,4R)-4-methyl-3-heptanol, das enantiomere eines pheromons des kleinen Ulmensplintkaefers (Scolytus multistriatus). Helv. Chim. Acta. 62:2829. |
| |
| Fuganti, C., Grasselli, P., Servi, S., and Zirotti, C. 1982. Synthesis of the enantiomeric forms of cis and trans 1-benzyloxy-2,3-epoxy butane and of (3S,4S)-4-methyl-3-heptanol. Tetrahedron Lett. 23:4269-4272. |
| |
| Fujisawa, T., Tajima, K., and Sato, T. 1984. Chirality transfer in the ester enolate Claisen rearrangement of (R)-1-methyl-(E)-2-butenyl hydroxyacetate and its application to the stereocontrolled pheromone synthesis. Chem. Lett. 1669. |
| |
| Hoffmann, R.W., Ladner, W., and Helbig, W. 1984. Stereoselektive synthese von alkoholen. XVIII. Synthese von (3S,4S)-4-methyl-3-heptanol und von (5S,6S)-anhydro-serricornin. Liebigs Ann. Chem. 1170. |
| |
| Koreeda, M., and Tanaka, Y. 1982. Stereoselective acyclic synthesis via allylmetals: an efficient synthesis of (�)-4methylheptan-3-ol, an aggregation pheromone of the smaller European elm bark beetle. Chem. Lett. 1297-1298. |
| |
| Matteson, D.S., and Sadhu, K.M. 1983. Boronic ester homologation with 99% chiral selectivity and its use in syntheses of the insect pheromones (3S,4S)-4-methyl-3-heptanol and exo-brevicomin. J. Am. Chem. Soc. 105:2077-2078. |
| |
| Matteson, D.S., Sadhu, K.M., and Peterson, M.L. 1986. 99% Chirally selective syntheses via pinanediol boronic esters: insect pheromones, diols, and an amino alcohol. J. Am. Chem. Soc. 108:810-819. |
| |
| Mori, K. 1977. Absolute configuration of (-)-4-methylheptan-3-ol, a pheromone of the smaller European elm bark beetle, as determined by the synthesis of its (3R,4R)-(+)- and (3S,4R)-(+)- isomers. Tetrahedron. 33:289-294. |
| |
| Mori, K., and Iwasawa, H. 1980. Preparation of the both enantiomers of threo-2-amino-3-methylhexanoic acid by enzymatic resolution and their conversion to optically active forms of threo-4-methylheptan-3-ol, a pheromone component of the smaller European elm bark beetle. Tetrahedron. 36:2209-2213. |
| |
| Nakagawa, N., and Mori, K. 1984. Synthesis of (3S,4S)-4-methyl-3-heptanol and its (3S,4R)-isomer employing asymmetric epoxidation coupled with regioselective cleavage of epoxides with trimethylaluminum. Agric. Biol. Chem. 48:2505. |
| |
| Narasaka, K., and Miwa, T. 1985. Highly enantioselective synthesis of anti (threo)-aldols by the asymmetric aldol reaction utilizing a chiral azaenolate. Chem. Lett. 1217. |
| |
| Pearce, G.T., Gore, W.E., Silverstein, R.M., Peacock, J.W., Cuthbert, R.A., Lanier, G.N., and Simeone, J.B. 1975. Chemical attractants for the smaller European elm bark beetle Scolytus multistriatus (Coleoptera: Scolytidae). J. Chem. Ecol. 1:115-124. |
| |
| Plummer, E.L., Stewart, T.E., Byrne, K., Pearce, G.T., and Silverstein, R.M. 1976. Determination of the enantiomeric composition of several insect pheromone alcohols. J. Chem. Ecol. 2:307. |
| |
| Pougny, J.-R., and Sinay, P. 1982. (3S,4S)-4-Methylheptan-3-ol, a pheromone component of the Smaller European elm bark beetle: synthesis from D-glucose. J. Chem. Res. 1:186-196. |
| |
| Rossi, R., and Marasco, M. 1980. Insect pheromones by asymmetric synthesis. Asymmetric synthesis of (S)-4-methyl-3-heptanone, the alarm pheromone of Atta texana, of (4S)-4-methyl-3-heptanol, the major aggregation pheromone component of Scolytus scolytus, and of (S)-3-methyl-2-heneicosanone, a structural analogue of a sex pheromone component of Blattella germanica. Chim. Ind. (Milano). 62:314. |
| |
| Sayo, N., Azuma, K.-I., Mikami, K., and Nakai, T. 1984. Acyclic stereocontrol via asymmetric [2,3]-Wittig rearrangement with high enantio- and erythro-selectivity and its use in the chiral synthesis of insect pheromones. Tetrahedron Lett. 25:565-568. |
| |
| Schlosser, M., and Fujita, K. 1982. A stereocontrolled synthesis of the pheromone 4-methyl-3-heptanol: novel and selective cc-linking reactions. Angew. Chem. Int. Ed. Engl. 21:309-310. |
| |
| Steiger, B., Wadder, L., and Scheffold, R. 1986. Sonnenenergie in der organischen chemie: photokatalytische elektrosynthese eines pheromone. Chimia. 40:93. |
| |
|
|







