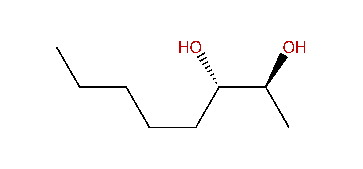
| Formula: | C8H18O2 |
| CAS#: | 37163-97-6 |
| MW: | 146.23 |
[MS]
[ NMR ]
[ Kovats ]
[Behavioural function]
|
|
Reference(s) for synthesis of (2S,3S)-2,3-Octanediol [2S3S-octanediol]
| Bergstad, K., Jonsson, S.Y., and Baeckvall, J.-E. 1999. A new coupled catalytic system for dihydroxylation of olefins by H2O2. J. Am. Chem. Soc. 121:10424-10425. |
| |
| Cainelli, G., Contento, M., Manescalchi, F., and Plessi, L. 1989. Catalytic hydroxylation of olefins by polymer-bound osmium tetroxide. Synthesis. 1:45-47. |
| |
| Jonsson, S.Y., Faernegardh, K., and Baeckvall, J.-E. 2001. Osmium-catalyzed asymmetric dihydroxylation of olefins by H2O2 using a biomimetic flavin-based coupled catalytic system. J. Am. Chem. Soc. 123:1365-1371. |
| |
| Katzenellenbogen, J.A., and Bowlus, S.B. 1973. Stereoselectivity in the reduction of aliphatic alpha-ketols with aluminum hydride reagents. J. Org. Chem. 38:627-632. |
| |
| Masaki, Y., Serizawa, Y., Nagata, K., Oda, H., Nagashima, H., and Kaji, K. 1986. Synthesis of chiral 1,2-diols and related compounds of biological activities via stepwise ring fission of 5-alkyl-6,8-dioxabicyclo[3.2.1]octane skeleton. Tetrahedron Lett. 27:231-234. |
| |
| Mori, K., and Otsuka, T. 1985. Synthesis of (2S,3S)-2,3-octanediol and (S)-2-hydroxy-3-octanone, the male sex pheromone of the grape borer Xylotrechus pyrrhoderus. Tetrahedron. 41:553-556. |
| |
| Nakata, T., Tanaka, T., and Oishi, T. 1983. Stereoselective reduction of alpha-hydroxy ketones. Tetrahedron Lett. 24:2653-2656. |
| |
| Pedrocci-Fantoni, G., and Servi, S. 1986. Simple synthesis of the two enantiomeric forms of erythro-octane-2,3-diol and 2-hydroxyoctan-3-one, proposed pheromones of Xylotrechus pyrrhoderus. J. Chem. Res. 199. |
| |
| Sakai, T., Nagagawa, Y., Takahashi, J., Iwabuchi, K., and Ishii, K. 1984. Isolation and identification of the male sex pheromone of the grape borer Xylotrechus pyrrhoderus Bates. Chem. Lett. 263. |
| |
|







